
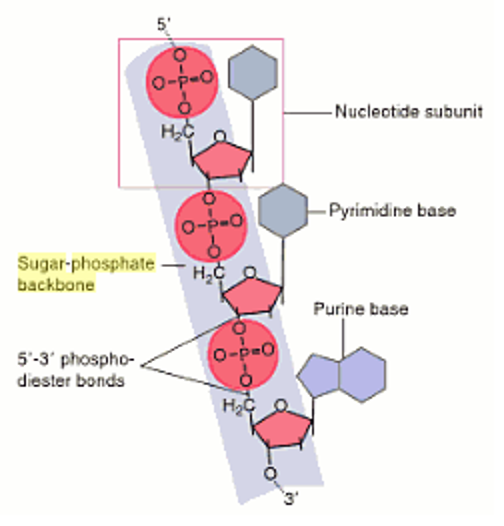
Sugar-phosphate backbone forms the structural framework of. These two sugars only differ by one -OH group being changed to an -H, but provides different capabilities for each molecule. The backbone is negatively charged and hydrophilic, which allows strong interactions with water. On on the other hand, the sugar in the backbone of RNA is called ribose. In DNA, the sugar involved is deoxyribose. However, their sugar phosphate backbone differs slightly.
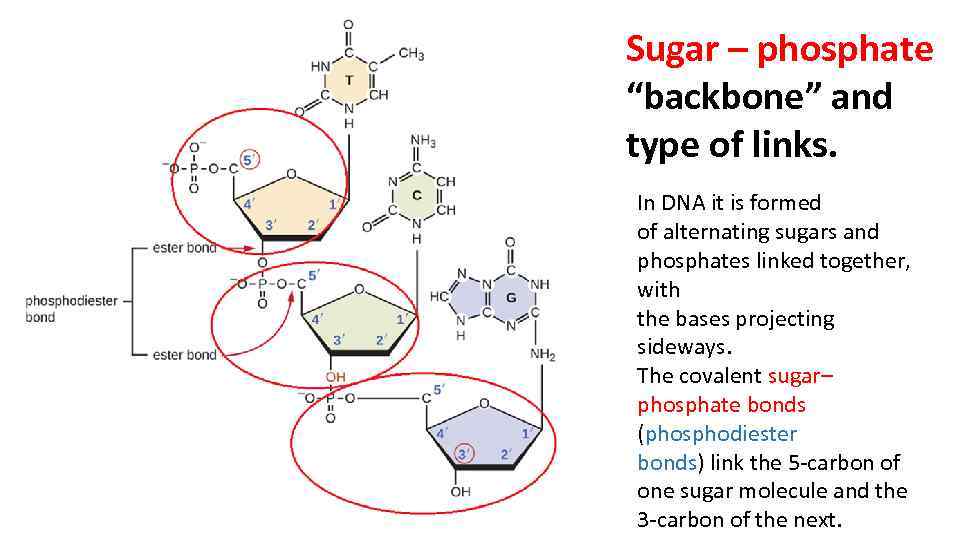
RNA and DNA are both examples of phosphodiesters and have a very similar structure. stacking interactions1,2 (electrostatic interactions and hydrophobic forces. One turn of this helix is 34nm long, the diameter of it is 2nm, and there are ten bases attached per turn at 0.34nm. conformation along the sugar-phosphate backbone in nucleotides can be. These features make DNA can repel water and would not hydrolysed and breakdown by the aqueous environment. DNA is very stable due to rungs of “ladder” is hydrophobic and phosphate sugar backbone of DNA is negatively charged. The purpose of this twisting is to protect the bases inside it, and prevent them from being damaged by the environment. one runs 3' to 5', the other run 5' to 3'. This is done by the sugar phosphate backbone twisting around itself in a coil. The genotype is determined by the sequence of bases.Figure 1 Diagram showing the sugar phosphate backbone of DNA, and the nitrogenous bases attached to it, forming a nucleotide Structure of DNAĭNA is wound into an right-handed double helix. It is this base sequence which forms the genetic code. This creates the twisting double helix structure of DNA.Īll cells store their genetic information in the base sequence of DNA. The two strands of DNA are antiparallel which means that one strand runs in a 5’ to 3’ direction and the other runs in a 3’ to 5’ direction. This makes sense: the sugars are very polar, and every phosphate group carries a. Sugar-phosphate backbone forms the structural framework of. The two pentose-phosphate backbones are at the exterior of the double helix. the 3' end (said as "3 prime end") at the deoxyribose end The backbone is negatively charged and hydrophilic, which allows strong interactions with water.the 5' end (said as "5 prime end") at the phosphate end Phosphate group Deoxyribose sugar Thymine base A number of hydrogen bonds Hydrophilic molecules, are those which has the tendency to dissolve readily in.These strong bonds form a sugar-phosphate backbone. These basic units are linked together to form strands by strong bonds between the deoxyribose sugar of one nucleotide and the phosphate of the next nucleotide. In proteins, the specific order of amino acids in a polypeptide (primary structure) determines the overall shape of the protein. DNA and RNA differ in structure and function. They always pair up in a particular way, called complementary base pairing: Each nucleotide has structural components: a five-carbon sugar (deoxyribose or ribose), a phosphate, and a nitrogenous base (adenine, thymine, guanine, cytosine, or uracil). Right: DNA double helix structure consisting of a hydrophilic sugar- phosphate backbone and hydrophobic nitrogenous base pairs. There are chemical cross-links between the two strands in DNA, formed by pairs of bases held together by hydrogen bonds. The nucleotides are identical except for the base, which can be one of four bases:


 0 kommentar(er)
0 kommentar(er)
